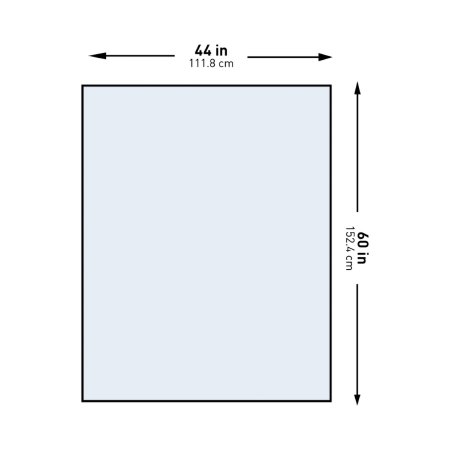
General Purpose Drape McKesson Half Drape Sheet 60 W X 44 L Inch Sterile
Product Specification Sheet
| Manufacturer | |
|---|---|
| UNSPSC | |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/general_drape_tear_sheet_2021-03.pdf |
| IsRetailItem | |
| Units of Measurement |